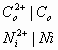金属可以看成是由离子和自由电子组成。
金属离子以点陈排列,电子在其间运动。如果我们把金属,例如锌片,浸入合适的电解质溶液(如ZnSO4)中,由于金属中
电极电位可表示为
式中
E:电极电位,V;
Eo:标准电极电位,V;
R:气体常数,8.31441J/(mol·k);
T:绝对温度,k;
n:参与电极反应的电子数;
F:法拉第常数,96486.7C/mol;
a:参与化学反应各物质的活度。
式(1.1)是电极电位的基本关系式。如果以常用对数表示,并将有关常数值代入,式(1.1)可写为
式(1.2)即著名的能斯特(W.H. Nernst)方程。
当溶液很稀时,活度可近似用浓度代替,上式可定为
如果电极反应为
根据式(1.2),在25℃时则有
金属离子活度
假定是Ag丝插入AgNO3中,则电极反应为
那么,在25℃时的电极电位为
如果电极体系是由金属、该金属难溶盐和该难溶盐的阴离子组成,如Ag-Agcl-Kcl电极体系,电极反应为
当存在Agcl时,银离子活度
代入(1.6)式可得
当
单个的电极电位是无法测量的,因为当用导线连接溶液时,又产生了新的溶液-电极界面,形成了新的电极,这时测得的电极电位实际上已不再是单个电极的电位,而是两个电极的电位差了。同时,只有将欲研究的电极与另一个作为电位参比标准的电极电位组成原电池,通过测量该原电池的电动势,才能确定所研究的电极的电位。原电池的电动势为
式中,E阴是阴极电极电位,E阳是阳极电极电位,Ej是液体接界电位,IR是溶液的电阻引起的电压降。可以设法使Ej和IR降至忽略不计,这样,上式可简化为E电池=E阴-E阳。如果E阴或E阳是一个已知的电极电位,那么,由测得的电池电动势减去已知的电极电位,即可求得另一个电极的电位。作为已知的电极电位的电极,可以采用标准氢电极(SHE),也可以采用作为二级标准电极的银-氯化银电极和甘汞电极。