Student
names:_________________________________________
CM 440/667
Practical:
Voltammetry Simulation
Students
who are scheduled to do the polarography prac prior to this date should attempt
the simulation before doing the prac - it will greatly enhance your
understanding of the prac.
The program Polar41c has been downloaded onto the computers
in the Department's computer lab. You can download a copy yourself (as a zip
file) from http://www.DrHuang.com and install it on your own computer.
In this exercise you will be given some information about
voltammetry and asked to predict the outcome of an experiment. You will then perform the experiment on the
simulator, compare the outcome with your prediction, and think about any
discrepancies. You will not
receive extra marks for making the 'right' prediction. Rather, I will be looking for evidence of
your engagement with the process of prediction, comparison, and
reflection. You may work in pairs or a
small group, discussing your predictions and any reasons for discrepancies
between them and the outcome of the simulation. Don’t feel too constrained by these notes – feel free to explore
the simulation along the way, doing additional experiments if you wish (a
couple of proformas are attached to the end of this document to record the
results of any such experiments.)
DC
Voltammetry
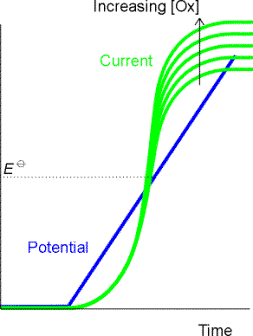
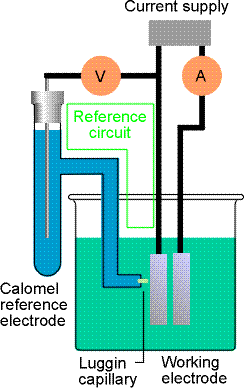
In voltammetry, an inert electrode
(eg., Pt, C, Hg) is placed in a solution containing electroactive species. (Polarography is a subset of voltammetry
that uses Hg electrodes.) The potential
of the electrode is changed as a function of time.
If an
electrochemical reaction occurs, electrons are transferred and a current
flows. The current is measured as a function
of the potential applied to the electrode.
A voltammogram is a plot of the current, i, on the vertical axis versus
the potential, E, on the horizontal axis.
Cathodic (reduction) currents are shown in the positive y-direction and
negative potentials are shown in the positive x-direction
In this series of experiments we are
looking at the two-electron reduction of Cu2+ to Cu. The Eo of the reduction is +0.34
V relative to the standard hydrogen electrode, or +0.10 V relative to the saturated
calomel electrode (which is more commonly used as a reference in voltammetry).
If we apply a potential of greater
(more positive) than 0.10 V, the reaction
Cu2+ + 2e
® Cu
will not occur. In voltammetry of metal ions, we start with
an applied potential well in excess (more positive) of the Eo of the
metal ion present and scan the potential in the negative direction as a linear
function of time. What do you think the
voltammogram (i versus E) will look like as the potential approaches and passes
the Eo? Remember there are
no marks for getting it right - just give it a go!
Load the program:
·
Start/ Programs/ Polar/ Polar
·
Maximise
Now run the simulation:
·
Input/ Techniques ® DC voltammetry
·
Input/ Mechanism ® A + 2e®B
·
Run/ Simulate
How does the simulated voltammogram
compare with your prediction? Did you
predict the gradual increase of current with voltage around the Eo? Current (coulombs per second) is a measure
of the rate of the electrochemical reaction. The reaction starts slowly at first but is driven faster as the
voltage is increased. Did you predict
the levelling off of the current or did you have the current increasing
infinitely? Can you offer an
explanation for the current plateau?
As Cu2+ ions near the
surface of the electrode react to form Cu (that is deposited on to the
electrode), the region close to the electrode is depleted of Cu2+
ions. We have
to wait for more Cu2+ ions
to diffuse from the bulk solution
(where they are in high concentration) to the electrode surface (where they are
in low concentration) before the reaction can continue. The rate of reaction,
and hence the current, becomes limited by the rate of diffusion of ions from
the bulk of the solution to the electrode surface, and the current plateaus.
There are a couple of parameters that
can be obtained from the voltammogram that are of interest to us. What do you think these might be? Draw them on a voltammogram:
Find out what they are:
·
Analysis/ Find Halfwave E
Can you see where these are on your
simulated voltammogram? You can put
gridlines in if you want:
·
Display/ Option ® Grid
The diffusion current , id,
is the (diffusion) limited current at the top of the voltammogram. The half-wave potential, E1/2, is
the potential at id/2.
What effect do you predict changing the
concentration of the Cu2+ ions will have on the voltammogram? Sketch a voltammogram for the 1 x 10-3
M solution you have already studied, and for a 2 x 10-3 M solution.
Now run the simulation:
·
Display/ Option ® Overlap (this enables the two
voltammograms to be displayed on the same plot)
·
Input/ Chemicals ® Canal(A) = 2e-3
·
Run/ Simulate
What has happened to id and
E1/2? Find out:
·
Analysis/ Find Halfwave E
You may well have expected to see a
change in E1/2 with concentration since this is what is seen in
potentiometry (as described by the Nernst equation). In voltammetry, E1/2 is unchanged by concentration,
and it is id that is concentration dependent. Try another couple of concentrations, then
plot id versus concentration in the space below. What do you notice?
The linear relationship between id
and concentration can be used as an analytical tool to determine the
concentration of an electroactive species in solution. But how do we know what electroactive
species is/are present? It's the value
of E1/2 that can indicate this.
As you know, redox couples have distinct reduction potentials. The half-wave potential, E1/2 is
very closely related to the Eo values.
What do you think that the voltammogram
for the reduction of Pb2+ (Eo = - 0.40 V vs. SCE) will
look like? Draw voltammograms for Cu2+
and Pb2+ on the same axes.
Now check your prediction
·
Display/ Option ® Overlap off
·
Input/ Chemicals ® Canal(A) = 1e-3
·
Input/ Instrument ® Eend
= -0.6 V
·
Run/ Simulate
·
Display/ Option ® Overlap on
·
Input/ Chemicals Eo = - 0.40
·
Run/ Simulate
·
Analysis/ Find Halfwave E
Was your prediction right? E1/2 can be used for
identification.
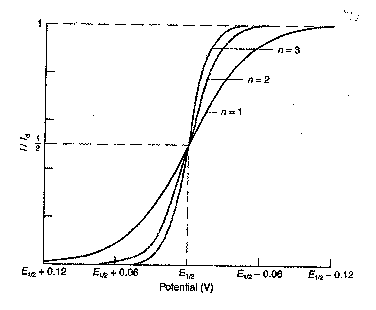
Next we look at the effect of changing
the number of electrons (n) involved in the reduction process. What do you predict will happen to the voltammogram
as you increase the number of electrons?
Will the current that flows increase, decrease, or stay the same? Will the diffusion limited current, id,
increase, decrease, or stay the same?
Will there be any effect on E1/2? Draw a series of voltammograms for n = 1, 2 and 3.
Now do a series of experiments and see
how your prediction measures up:
·
Display/ Option ® Overlap off
·
Input/ Techniques ® DC voltammetry
·
Input/ Chemicals ® Eo = 0.1
·
Input/ Instrument ® Eend
= - 0.3 V
·
Input/ Mechanism ® A + 1e®B (default
setting)
·
Run/ Simulate
·
Display/ Option ® Overlap on
·
Input/ Mechanism ® A + 2e®B
·
Run/ Simulate
·
Input/ Mechanism ® A + 3e®B
·
Run/ Simulate
·
Analysis/ Find Halfwave E
Describe the effect on the shape of the
voltammogram, the diffusion-limited current, id, and the half-wave
potential, E1/2? Is that what you were expecting?
The diffusion limited current, id,
increases linearly with n. The diagram
opposite is normalised (i/id) and does not show this.
The shape of the voltammogram is given
by the equation:
E = E1/2 + (0.0592/n)*log [(id - i)/i]
So, as n is increased the voltammetric
wave (i versus E) becomes steeper.
What effect might varying the diffusion
coefficient, D, of the metal ion have?
(The diffusion coefficient could change as a result of a change in temperature or a change of solvent.) An increase in the diffusion coefficient
means that the metal ion can move from the bulk solution to the surface of the
electrode faster. What effect would
this have on the limiting current, id? What effect would there be on E1/2? Draw a series of voltammograms for which D =
1x 10-5, 4 x 10-5, and 9 x10-5 cm2/s.
Now do the experiment:
·
Display/ Option ® Overlap off
·
Input/ Mechanism ® A + 2e®B
·
Input/ Chemicals ® Canal = 1e-3
·
Input/ Chemicals D =
1e-5 (default setting)
·
Run/ Simulate
·
Display/ Option ® Overlap on
·
Input/ Chemicals ® D = 4e-5 for A. Keep D
for B at 1e-5.
·
Run/ Simulate
·
Input/ Chemicals ® D = 9e-5 for A. Keep D
for B at 1e-5.
·
Run/ Simulate
·
Analysis/ Find Halfwave E
Can you
see a relationship between id and D? What is it? Is it what
you expected?
You were probably expecting id
to increase as you increased D, but you would probably not have expected the D1/2
relationship. More about that in lectures.
Were you surprised by the dependence of
E1/2 on D? Try plotting E1/2
versus log D on the grid overleaf. What
do you conclude?
E1/2
depends on log D. Again, more in
lectures.
Are there any other experiments that you would
like to do? A couple of proformas are
attached to the end of this document to record your aims, settings,
predictions, results and discussions.
Normal
Pulse Voltammetry
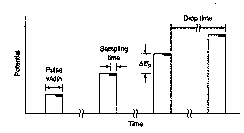
The current is sampled near the end of
the pulse when the Faradaic current (due to the reduction of Cu2+)
is large compared to the charging current (due to the migration of the
electroinactive supporting electrolyte ions in response to the charging of the
electrode) – ie., the signal-to-noise ratio is largest.
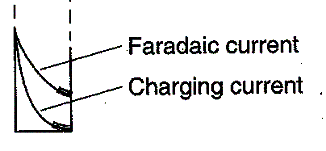
What do you predict that the resulting
voltammogram will look like? Draw a DC
voltammogram and the corresponding NP voltammogram.
Check out your prediction:
·
Display/ Option ® Overlap off
·
Input/ Chemicals ® D = 1e-5
·
Run/ Simulate
·
Display/ Option ® Overlap on
·
Input/ Techniques ® Normal pulse voltammetry
·
Run/ Simulate
·
Analysis/ Find Halfwave E
Were you expecting a similar shape
wave? Has E1/2 moved? How do the id's compare? Because the current is sampled near the end
of the pulse when the Faradaic current (due to the reduction of Cu2+)
is large compared to the charging current (due to the migration of ions in
response to the charging of the electrode), the signal is increased (as you can
see). But also the noise is decreased
and hence the signal-to-noise ratio is improved by a factor of about 10. The detection limit of NP voltammetry is 10-6
M, compared with 10-5 M for DC.
Differential
Pulse Voltammetry
In differential pulse (DP) voltammetry,
small pulses are superimposed on a linear voltage ramp.
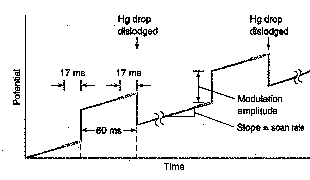
Current is measured before and after
the application of the pulse and the difference in current is recorded as a
function of the applied voltage. The
resulting voltammogram is nearly the derivative of a DC voltammogram. Can you predict the shape of the
voltammogram? Consider the size of Di as you move in small, constant DE steps along a DC polarogram. Where is Di
a maximum?
Check your prediction:
·
Display/ Option ® Overlap off
·
Input/ Techniques ® DC voltammetry
·
Run/ Simulate
·
Display/ Option ® Overlap on
·
Input/ Techniques ® Differential pulse voltammetry
·
Run/ Simulate
·
Analysis/ Find Peak
Did you predict the peak shape? What peak parameter do you think is most
likely to be used in place of E1/2 to identify an electroactive
species? What parameter do you expect
to be related to the concentration of the electroactive species? Indicate these parameters on a voltammogram.
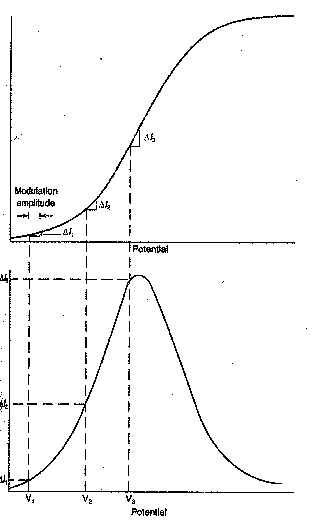
Run a series of experiments to test
that the peak current, ip, and the peak area are proportional to concentration,
c. Graph ip and peak area versus c.
The magnitude of the pulse that is superimposed on the DC ramp in
DP voltammetry can be changed. What
effect would increasing the size of this voltage pulse have on driving the
reaction and hence on the current?
Might any other peak parameters be affected? Sketch some voltammograms:
Well, try it and see:
·
Display/ Option ® Overlap off
·
Input/ Chemicals ® Canal = 1e-3
·
Input/ Instrument ® Epulse = - 0.05 (default
setting)
·
Run/ Simulate
·
Display/ Option ® Overlap on
·
Input/ Instrument ® Epulse = - 0.02
·
Run/ Simulate
·
Input/ Instrument ® Epulse = - 0.01
·
Run/ Simulate
·
Analysis/ Find Peak
You were probably expecting to see an
increase in the signal, but what else do you notice? Estimate the peak width (in volts) at half-height. To help you do this, you could insert
gridlines and/or print the voltammograms and measure the peak widths at half
height with a ruler. What do you
notice? What implications does this
have for resolution of neighbouring peaks?
DP voltammetry allows an even greater
increase in the signal to noise ratio and the detection limit is of the order
of 10-7 M. Because it is
easier to distinguish partially overlapping peaks than partially overlapping
waves, resolution is improved (provided too large a pulse is not used) from 0.2
V for DC and NP to 0.05 V for DP voltammetry.
Square Wave Voltammetry
In square wave (SQ) voltammetry, an
alternating (positive, negative, positive, negative….) wave that is square in
shape is superimposed on a voltage staircase.
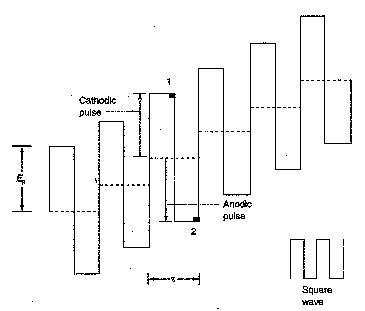
The reverse (anodic) pulse causes
re-oxidation of the product (Cu(s)) of each forward (cathodic) pulse. The voltammetric signal is the difference
between these two signals and hence will only be recorded when significant
amounts of the oxidised (Cu2+) and reduced forms (Cu(s)) are
present. Can you predict the shape of
the SQ voltammogram?
Try it and see:
·
Display/ Option ® Overlap off
·
Input/ Techniques ® Square Wave voltammetry
·
Run/ Simulate
Square wave voltammetry is about 5
times more sensitive than differential pulse.
It is also about 100 times faster - an entire square wave voltammogram
can be obtained in less than one second.
It can be used for the detection of compounds emerging from a liquid
chromatograph. Compounds are
distinguished by their retention times and E1/2 and are quantified
by peak height or area.
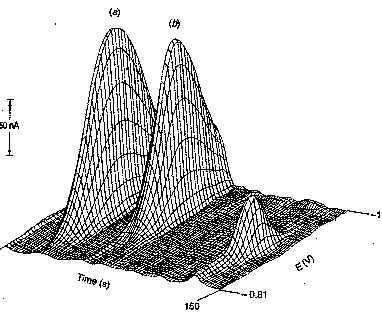
Stripping Analysis
In stripping analysis, Cu2+
is concentrated onto the electrode (as Cu) by reduction at a potential more
negative (why?) than E1/2.
The copper metal is then stripped off the electrode by reversing the
voltage - ie., sweeping in a positive
direction - driving the oxidation reaction.
The current measured during the oxidation is related to the amount of Cu
initially deposited which, under a given set of experimental conditions, is
related to the concentration of Cu2+. Predict the shape of a DC stripping voltammogram. How will the current measured compare with
that for a normal DC voltammogram?
Think about both the signs and magnitudes of the currents.
Run the simulation:
·
Display/ Option ® Overlap off
·
Input/ Techniques ® DC voltammetry
·
Run/ Simulate
·
Display/ Option ® Overlap on
·
Input/ Instrument ® Pre-concentration on. Note that the potential will be held at -
0.3 V for 600 s before being reversed and swept in the positive direction.
·
Run/ Simulate
·
Analysis/ Find Halfwave E
Stripping is the most sensitive of
voltammetric techniques because the analyte is concentrated from dilute
solution. The longer the period of
concentration, the more sensitive the analysis. Confirm this:
·
Input/ Instrument ® tpre = 1200 s
·
Run/ Simulate
·
Analysis/ Find Halfwave E
Detection limits are ca. 10-10
M
Linear Sweep Voltammetry
Linear sweep voltammetry (LSV) is
similar to DC voltammetry - a linear voltage ramp is applied to the electrode -
except that the rate of sweep is much greater. Do you expect the LS voltammogram to be the same as the DC? Think about what would happen at the surface
of the electrode as the voltage is rapidly increased. Would new Cu2+ ions have sufficient time to move from
the bulk solution to replace those that are reduced? Sketch the waveform that you expect for LSV.
Now do the experiment
·
Display/ Option ® Overlap off
·
Input/ Techniques ® DC voltammetry
·
Input/ Instrument ® Pre-concentration off
·
Run/ Simulate
·
Display/ Option ® Overlap on
·
Input/ Techniques ® Linear sweep and cyclic voltammetry
·
Run/ Simulate
·
Instead of levelling off at the top of
the LSV wave, the current decreases as the voltage is further increased. This happens because Cu2+ ions
become depleted near the surface of the electrode and movement from the bulk solution
is too slow to replenish them.
Perform a series of experiments to
confirm that the peak current, ip, is proportional to
concentration. Plot an appropriate
graph below.
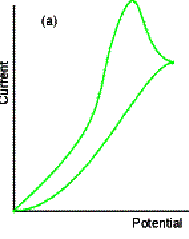
Cyclic Voltammetry
In cyclic voltammetry (CV), the LSV
voltage ramp is reversed to bring the potential back to its initial value. When the potential becomes sufficiently less
negative, Cu is oxidised back to Cu2+. The process of reduction and re-oxidation can be repeated many
times.
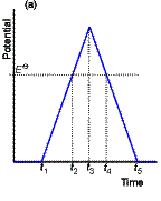
Predict the shape of the resulting
waveform:
Run the experiment
·
Display/ Option ® Overlap off
·
Input/ Techniques ® Linear sweep and cyclic voltammetry
·
Input/ Instrument ® Set "scan" to cycle
·
Run/ Simulate
Did you predict the anodic and cathodic
waves? Did you predict that ipa
= - ipc? Did you predict the
separation of the voltages at which the anodic and cathodic peaks occur? For a reversible reaction (one that is fast
enough to maintain equilibrium concentrations of reactant and product at the
electrode surface):
Epa
- Epc = 0.057 / n
where n is the number of
electrons. Is the Cu2+ + 2e ® Cu(s)
reaction reversible? Check it out:
·
Analysis/ Find peak
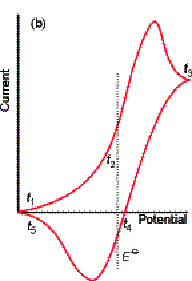
For an irreversible reaction, the peaks
are more drawn out and separated. Where
the oxidation is very slow, no anodic peak is seen.
Additional Experiments
Aim:
Settings:
Prediction:
Results:
Discussion:
Additional Experiments
Aim:
Settings:
Prediction:
Results:
Discussion: